If you want to get a balanced and healthy diet what information do you turn to right now? You have many choices. The simplest option would be to go online and follow a diet trend out there. The more complex option, if you’re really really savvy about nutrition, can be to follow the recommended dietary allowance (RDA) values for nutrients set out by the National Academy of Sciences.
Here’s the thing. These intake values, though updated regularly, are part of a system that’s over 50 years old (estd. in 1968) and considers individuals as statistical points on a graph that's normally distributed. The Dietary Reference Intakes (DRI) system has its flaws.
DRIs can neither provide precise quantitative assessments of the adequacy of diets of individuals nor be used to exactly assess nutritional status.
We now have evidence that nutrients intake can depend on your unique genetic makeup. So why not improve upon the system and make it potentially work better by using insights from your DNA?
Going beyond the Dietary Reference Intakes system
We now know that genetic disorders like hereditary hemochromatosis, fatal familial insomnia and increased breast cancer risk due to certain gene variants are very real.
Having a genetic variant doesn’t mean that you’ll end up with the disease but the probability of its occurrence goes up. The higher risk combined with a bad diet and lifestyle can actually result in the disease.
This extends to other conditions too - lactose, gluten and histamine intolerance, and conditions like hay fever can be genetically driven. There are also other factors at play like your microbiome and the number of exposures you have had to certain foods. However, genes can be a useful starting point considering that the cost of DNA testing has been falling around the world .
Going on a specific “diet” is akin to gambling
What if you decide to go the other route and decide to get on the bandwagon of a specific type of diet? Let’s take the example of veganism.
When you stick just to plant based foods without understanding the true macro and micro composition of your food, it can potentially harm you in the long run. The issue here is that when you stick to very specific food groups you are highly likely to become nutritionally deficient in one or more critical micro-nutrients. For instance, if you’re on a plant based diet you won’t get enough of Omega3 fatty acids - a nutrient that’s important for brain function.
EPA and DHA is mostly present in seafood and our own bodies can’t make it. Fish feed on plant based ALA - a plant based Omega 3 fatty acid, and convert it to EPA and DHA, which we then consume.
In most cases the absorption of plant based ALA is likely to be very low - moreover it’s a shorter-chain polyunsaturated fatty acid that isn’t as effective in cell growth. What if on top of this you also have a genetic mutation that lowers your ability to convert ALA to EPA and DHA?
If you’re on a meat based ketogenic diet, how do you know you are having the right combination of fats - especially saturated fats?
Personalizing nutrition based on genetics
Research shows that providing personalized nutrition information based on diet, lifestyle and genotype “produced larger and more appropriate changes in dietary behaviour than a conventional approach.” The link between your diet and chronic conditions like Type II Diabetes, Alzheimer’s, Heart disease etc… is well established. If chronic illnesses are indeed linked to diet then why not use that lever to derisk? You’re eliminating one big factor on top of all the other complex variables.
It’s really simple.
Your genetics can dictate your nutritional absorption and your genetic disease risk can influence the need for more or less of certain nutrients. Ergo, you need more or less of different macros and micronutrients based on your unique genetic makeup. On top of this, what is beneficial for you can be driven by other factors like food allergies, intolerances and health risks posed by conditions like iron overload.
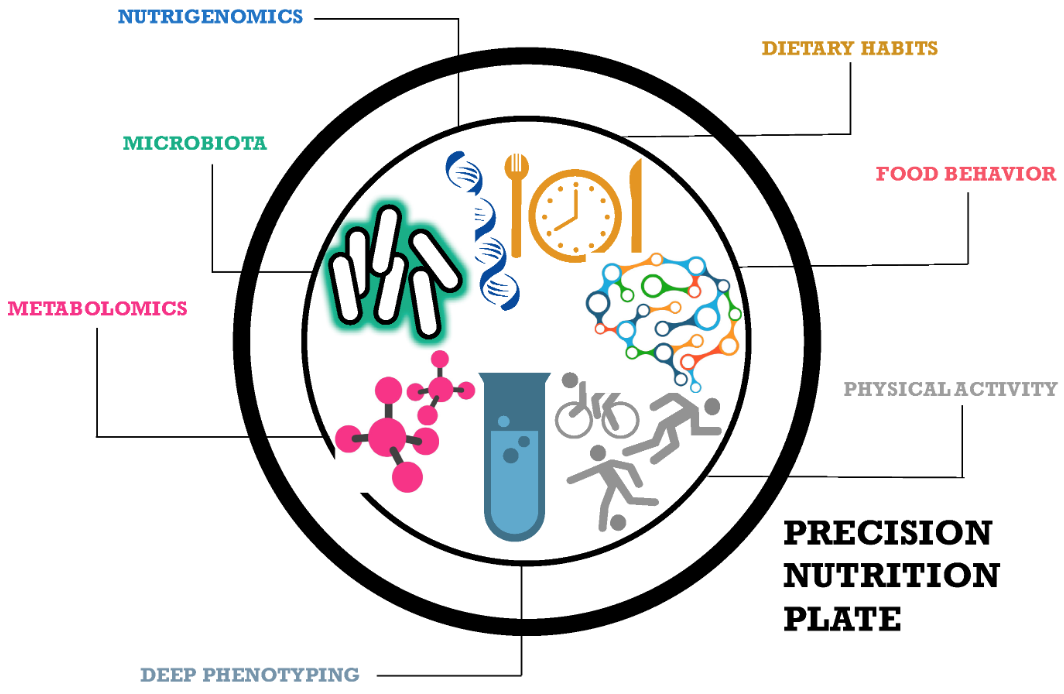
Image sourced from here.
Let’s explore the science behind nutrigenomics, nutrigenetics and epigenetics first before we dive into individual genes.
Nutrigenetics
The idea that the nutrients we eat can interact with the physiological functions and metabolism of the body at the molecular levels, is termed as nutrigenetics.
It involves the effect of dietary components on different gene variants. This genetic variation in individuals governs the dietary response of an individual towards foods and how it influences the digestion, absorption, and assimilation of the nutrients present in the food.
The study of nutrigenetics has become a developing area of research and gene-nutrient interactions has garnered the interest of a lot of scientists all over the world. Research is showing that various monogenetic and polygenetic diseases are caused by adverse effect of certain nutrients that may be unsuitable for that person based on his distinct genetic makeup.
Nutrigenomics
The influence of nutrients in food on the expression of various genes is termed as nutrigenomics.
This field provides a detailed insight on the effects on our genes of various macro- and micronutrients that we eat.
Like other biotic and abiotic environmental factors that influence our health and well-being, food is now considered as the most vital environmental factor that can have positive or negative effects on our body. In some cases, if the nutrient is not in harmony with our genetics and metabolic system, then it proves to be a nuisance and can deteriorate our bodily functions.
Many researches have linked diabetes, atherosclerosis, cancer, hypertension with the amount of calories intake, type of fats, and types of vitamins. Various dietary interventions related to nutrition requirements, genotype and nutritional status are being used to control and prevent various metabolic syndromes, cardiovascular diseases and cancer.
Interaction between Nutrigenomics and Nutrigenetics
Nutrigenetics and Nutrigenomics are similar and yet different terms.
Nutrigenomics is the interaction of nutrients with our genome while nutrigenetics is the variations in the response to various nutrients present in the food according to our distinct genetic makeup.
The genetic responses (nutrigenomics) involve the effect of food on mutation selection, gene expression, evolution of genome, chromosome stability, protein synthesis, metabolic pathways and chronic diseases. The nutritional responses on the other hand include the effect of dietary components on the absorption of nutrients, nutrient tolerance and food allergies (Figure 1).
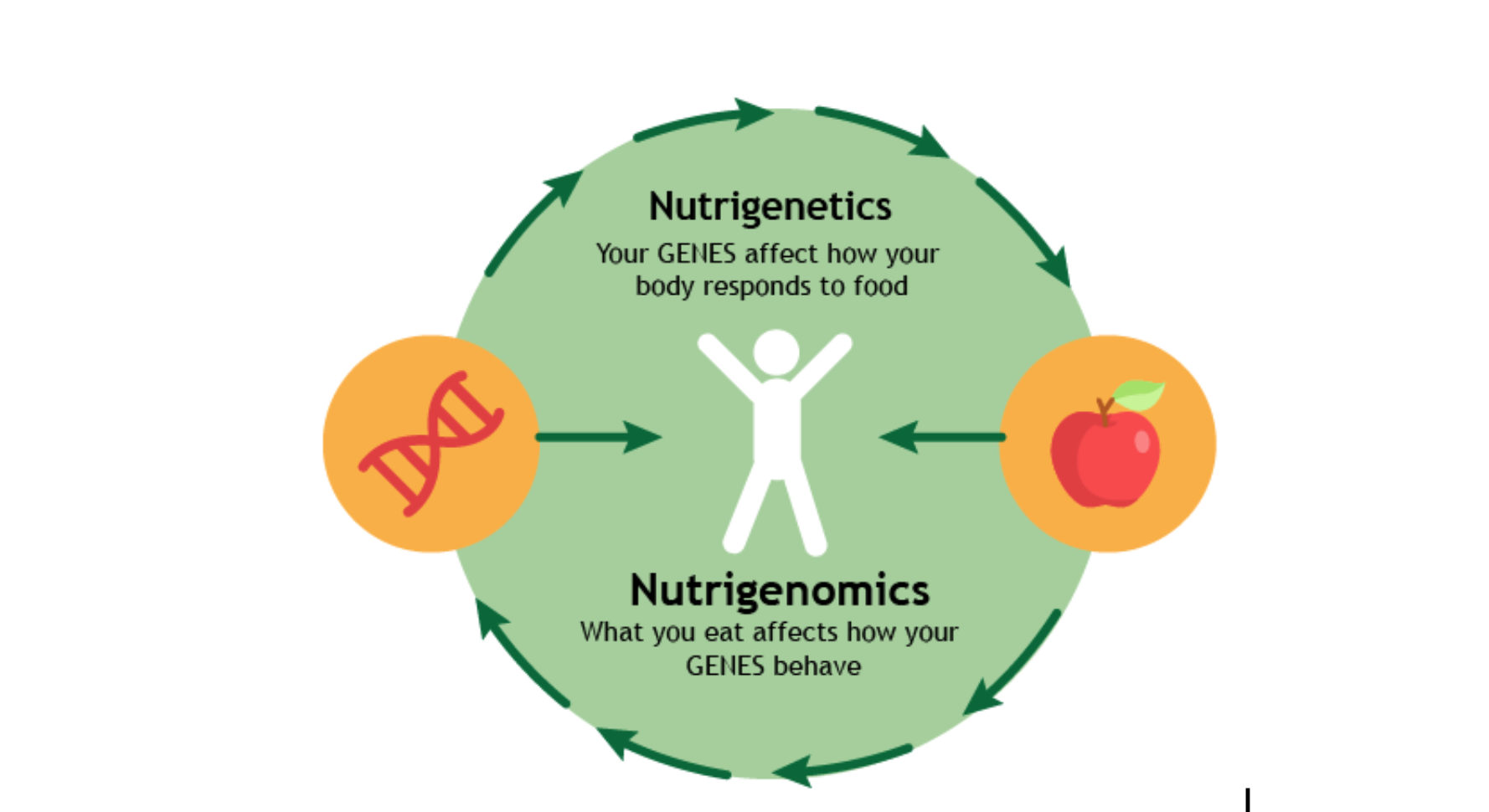
Figure 1: The interconnection between nutrigenomics and nutrigenetics
Epigenetics and nutrition
The term epigenetics is used for the changes that affect the expression of genes without causing the DNA sequence to change.
These DNA changes via epigenetics can have long term effects as they can be carried over the generations and lead to transfer of non-genomic epigenetic alterations.
There are various environmental factors that primarily act as regulators of epigenetic changes. Various, environmental pollutants/toxins, drugs, physical activity, behavior, stress, smoking and working habits are some of the environmental and lifestyle factors that influence epigenetic processes including, histone acetylation, and DNA methylation.
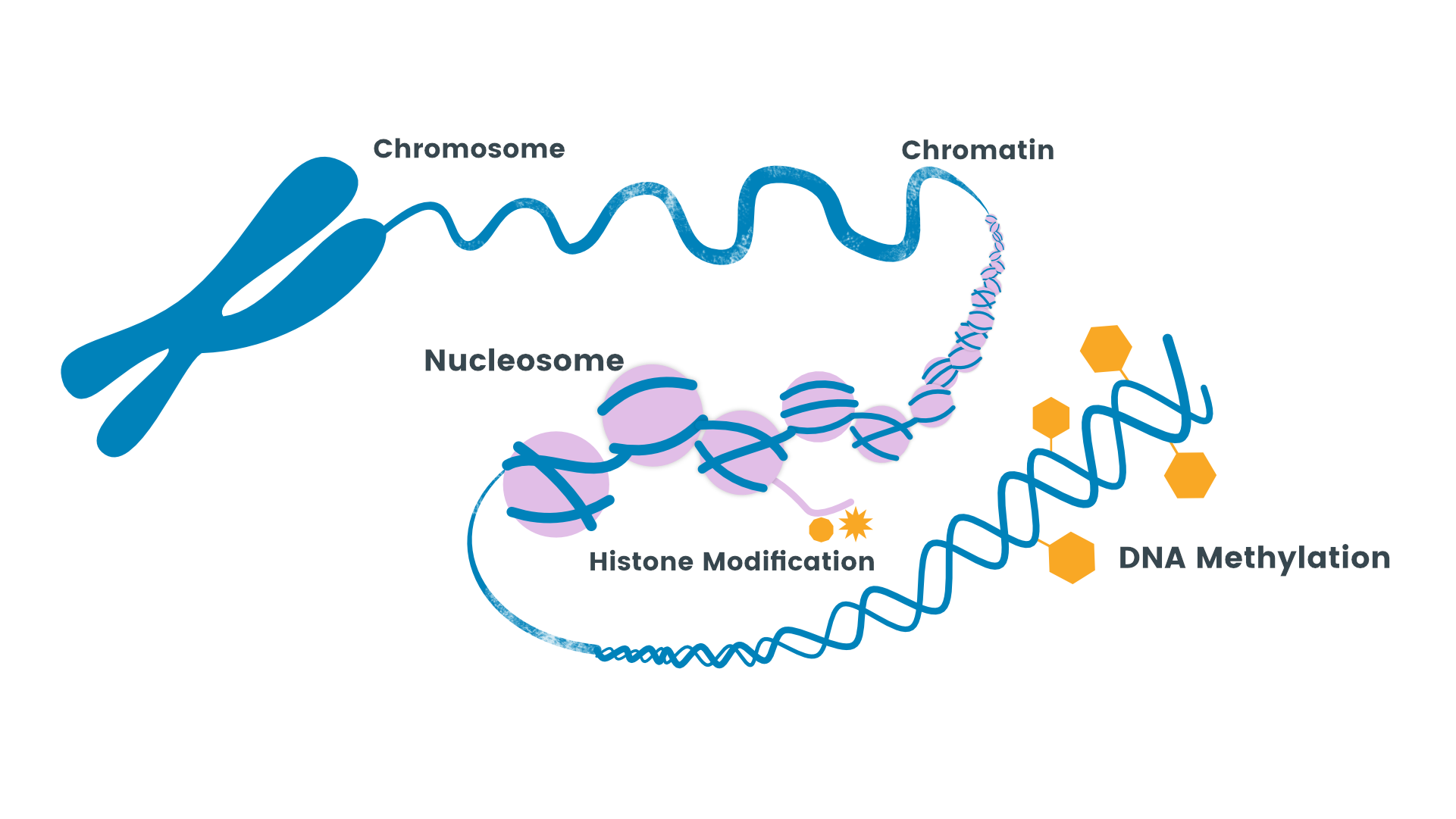
Epigenetics research
How environmental cues are biologically integrated and transgenerationally inherited, is still poorly understood. The effects of changes in abiotic environmental factor on nematodes gene expression over generations, was studied and it was found that when a transgene was introduced in nematodes and were exposed to high temperatures, the introduced gene was turned on and led to the glowing of the organisms. This feature was transferred to the next generation and despite of absence of exposure to high temperature, the nematodes carried the glowing feature for upto seven generations which shows that the epigenetic changes induced due to temperature factor in the parent generation were carried to next generations without changes in the actual DNA sequence.
Such epigenetic events that can influence the gene expression can occur though bioactive components of the food.
There are various crucial processes in the cell that can be regulated though epigenetic effects of various nutrients, such as apoptosis, DNA damage, cell cycle control, and aging. Some of the widely studied nutrient groups that have distinct epigenetic effects on the cells include carotenoids, indoles, beta-glucans, n-2 fatty acids, flavonoids, and isothiocyanates. It has been reported that DNA methylation, that is one of the most widely occurring epigenetic mechanisms in the cell, can be regulated or changed by dietary components such as zinc, arsenic, nickel, fiber and alcohol.
Effects of micronutrient deficiencies on the human genome
Humans require around 40 micronutrients that are essential for the maintenance of good health. These nutrients provide all the essential building blocks of vital proteins and molecules in the body. A wide variety of dietary factors directly affect the DNA and the genome and influence the metabolic system of the body.
The inadequacy of certain micronutrients can adversely affect certain tissues and functions of the body, for example deficiency of vitamin D, folate, vitamin B and E can lead to bone defects, cancer and cardiovascular disease, respectively.
The damage of nutrient deficiency penetrates down to the DNA and genomic levels, and various nutrients have been identified as direct regulators of the genome structure and functions. For instance, vitamin D plays a role in apoptosis process of cancerous and abnormal cells and it’s deficiency can lead to formation of DNA lesions and increase DNA damage. Similarly, vitamin B2, B6, B12 have protective effects on DNA and inadequate level of these vitamins in the body can lead to an increase in chromosomal breaks and misincorporation of uracil in the DNA. Iron is important for DNA and genomic integrity and iron deficiency has also been linked with reduced DNA repair ability and increased oxidative damage to the DNA.
Genetics, nutrition and diseases
The human genome is affected by dietary components. Individuals who are predisposed to a certain disease can experience metabolic diseases which are triggered when their body is burdened with the wrong nutrients!
Let's look some of the latest research that has surfaced on how genetics and nutrition are tied together.
Saturated fat risk - ApoE genotype
The apolipoprotein gene (ApoE) is involved in the metabolism and transport of lipids and fats in the body. There are three variants/alleles of these genes and the variant ε4 is prone to developing coronary heart disease. The reason behind this increased risk is the inability of ε4 ApoE gene variant to metabolize high-fat diet.
This variant of ApoE is also linked with late-onset Alzheimer’s disease (AD) and is considered as the most crucial determinant of genetic predisposition to AD. The ApoE gene is responsible for transport of lipids in the brain along with other organs and failure to do so as happens in the APOE ε4, causes an increased level of brain amyloid-beta (Aβ), which contributes to the pathology of AD.
Various measures have been taken under clinical practices to prevent AD and these primarily involve nutrigenomic, pharmacogenomic and lifestyle changes of individuals who are at risk of developing AD.
Vitamin D metabolism
Vitamin D primarily regulates the homeostasis of calcium and maintain the integrity of bone.
However, it has been found that this nutrient has a genome wide effect as it has been found to regulate the expression of anywhere from 291 to over a 1000 genes.
Some of these genes whose expression was greatly increased due to the presence of vitamin D include the genes involved in apoptosis, immune function, health shock response genes, mitochondrial translation gene and DNA repair genes.
There were some genes whose expression was decreased or were down regulated by the increased serum level of vitamin D. These include certain transcriptional regulators, and members of the histone protein family.
Vitamin D levels have long been considered as important regulators of immune system and may provide protection against developing certain diseases such as Type 1 diabetes and multiple sclerosis.
Vitamin D promotes and maintains the health of brain cells, and was recently recognized as a neuro-steroid. It is metabolized to its active form in the glial and neuronal cells of the nervous system and plays an important role in cognitive abilities and provides protection against neurodegenerative diseases, depression, and various kinds of dementia.
The vitamin D receptor (VDR) gene is now considered as an important genetic marker to determine your predisposition to various diseases.
Weight gain tendencies
The metabolism of carbohydrates is one of the most vital processes in the body and any kind of defect or malfunction in this process can lead to an increased risk of developing various metabolic diseases. For instance, obesity has become one of the most challenging and widespread disorders in the world.
It has been reported that people who carry variants of PLIN1 are at an increased risk of obesity - they gain weight around the waist if they consume less than 144 grams of complex carbs.
Evidence suggests that SNPs in the FTO gene can cause increased energy intake and higher BMI. In studies, those carrying the obesity risk A allele of rs9939609 exhibited overall increased food intake, fat consumption and impaired satiety.
Type II Diabetes Risk
Various genes have been associated with Type II diabetes (T2D) making it a polygenic health condition. The presence of genetic markers are crucial indicators of risk of developing T2D in some stage of life. Some of these genes are TCF7L2, CDKAL1 etc. - they encode proteins that regulate the insulin and glucose production.
The presence of these genes along with certain environmental factors and lifestyle increases the risk of developing Type-II diabetes. Meal-timing, glycemic index of foods and other factors like lack of exercise, alcohol, smoking, poor diet and unhealthy lifestyle can lead to increased type-II diabetes risk.
Iron overload risk and Hemochromatosis
Iron is one of the most significant nutrients for the body. However, if the level of iron in the body is increased beyond a healthy normal value, it can have toxic effects on the organs and body function. This unwanted build-up of iron in the body is termed as iron overload and clinically this condition is known as hemochromatosis.
Hemochromatosis, like other metabolic diseases, also have a genetic aspect and involves the mutation in the HFE gene. HFE gene encodes the HFE protein that functions to regulate the uptake and absorption of iron, especially in the liver cells. If mutation, C282Y is present in the HFE gene of an individual, then the person is considered to be at a high risk of developing hemochromatosis. The actual incidence rate of hemochromatosis among carriers of this gene is very low but it is always good to be on the safer side.
The decreased consumption of iron rich food, and vitamin C-containing food is crucial for avoiding complications caused by iron overload.
DNA methylation and folate deficiency
The methylenetetrahydrofolate reductase (MTHFR) enzyme is considered as a vital enzyme that is involved in various metabolic processes including methylation and metabolism of folic acid and folate.
Methylation of DNA is an important gene regulatory process and carries out the switching on and off genes and involved in the DNA repair process. Methylation is essential for the conversion of folic acid and folate into their active form that is metabolized by the cell.
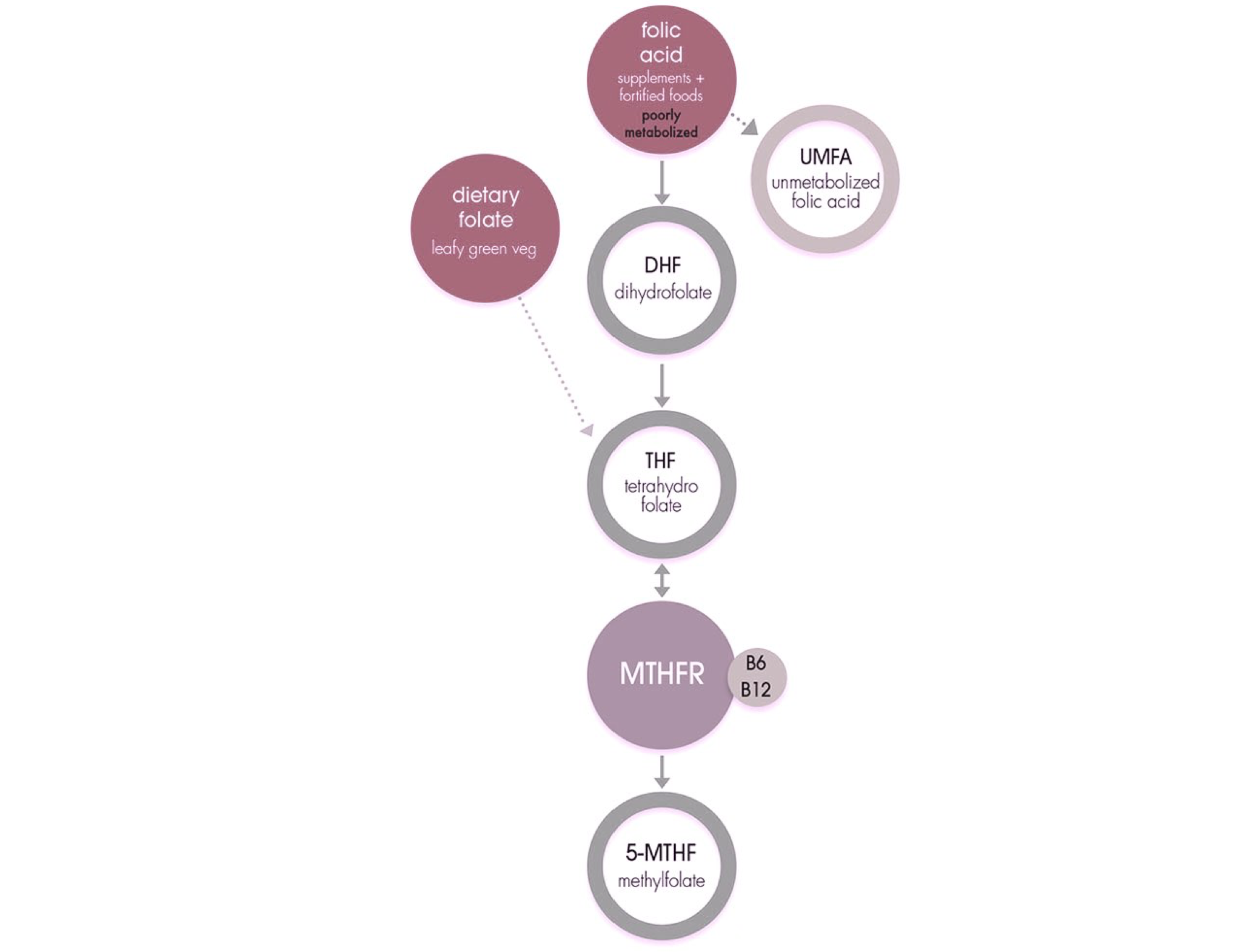
The role of MTHFR in folate cycle
There are two main mutations of the MTHFR gene, C677T and A1298C, that lead to abnormal function or complete absence of the MTHFR enzyme.
The presence of mutation in MTHFR gene lead to folate deficiency and this has a major health implication as folate is required for the normal metabolic processes of the cell.
Moreover, the deficiency of MTHFR enzyme also affects the metabolism of homocysteine (it plays a role in processing amino acids) which leads to increased risk of various other diseases, including stroke and other cardiovascular disorders.
In order to cope with folate deficiency in the population, the use of folic acid supplement has become a common practice and in countries like USA, Australia and Canada, where the wheat flour is fortified with folic acid. The question is how much do you need? And this comes down to the specific variants you carry.
Intolerances and Food Allergies
The fact that not all nutrients present in the food are equally beneficial for everyone has become the basis of the science behind nutrigenomics. In present days, various allergies triggered by components of the food have been identified and the prevalence of these food allergies is increasing in developing and developed countries.
The most common food allergens include peanuts, cow’s milk, eggs, nuts, soy, fish and wheat.The culprit behind the development of allergies is our own immune system. The cells of the immune system recognize certain food proteins as a threat or an antigen and elicit a detrimental response towards the harmless substance.
Gluten allergy is considered as the most significant food allergies and is aggravated by the consumption of wheat proteins.
Celiac disease is the most harmful implication of gluten allergy, where the immune system is triggered by gluten and the cells of the immune system targets the mucosal villi of small intestine. This autoimmune targeting of gastrointestinal lining lead to serious health damage as it decreases the absorption capacity of the small intestine.
Individuals with Celiac disease are advised to refrain from consuming wheat and wheat-based products so that their intolerance to gluten can be kept under check.
Similarly, with lactose intolerance, the sugar present in milk and dairy products lead to a negative immune response and damage the intestinal lining. Lactose intolerance is mostly genetic and can be inherited in infants and can develop in later stages of life due to reduced production of the enzyme lactase, that breakdown lactose.
Avoiding milk or consuming medicated milk that lack lactose is recommended for individuals with lactose intolerance. Other than food allergies, other allergies like hay fever, eczema are becoming a matter of concern for public health. It has been found that various genetic mutations that affect the DNA methylation and immune system regulators, are important genetic markers for allergic rhinitis, asthmas and eczema.
Link between Exercise and Gene expression
Exercise is becoming one of the most surprising and effective methods for the improvement of general wellbeing. Recent studies have focused on the genetic effects of exercise on the metabolic and structural features of the body. It has been reported that exercise has a positive effect on the expression of genes related functioning of skeletal muscles.
There is an integrative system of regulation of gene expression that is primarily controlled by exercise, which act as an epigenetic factor and triggers changes like DNA methylation, micro-RNAs and phosphorylation and acetylation of histone proteins for the regulation of various genes.
Other genes that are upregulated in muscles that have been subjected to regular exercise include metabolic priority genes and mitochondrial genes. The metabolic genes were found to be involved in the regulation of blood glucose levels and muscle glycogen level and aided in providing the necessary food for the exercise. While, mitochondrial genes that were expressed ensured the production of energy from the food. These genes make the muscles strong, prepare the body to endure physical stress, and exercise revamps the metabolic structure of the muscles and the body.
Evolutionary studies suggest that sedentary lifestyle and lack of physical activity can cause deteriorating effects on human health. And present studies support the idea that maintain a healthy lifestyle with proper involvement of exercise and physical activity can improve our brain function, benefit our organs, boost the immune system and provide protection against various diseases, such as diabetes, arthritis and cardiovascular disorders.
Moving away from a one size fits all approach
The importance of diet and nutrients for the health and wellbeing of humans and the science of nutrigenomics have paved the path for tailored or precision nutrition. Several metabolic disorders and diseases can be prevented or controlled through the use of customized nutrition. Precision nutrition plays a distinct role at different levels, including metabolic, genotypic and lifestyle patterns of an individual (Figure 3).
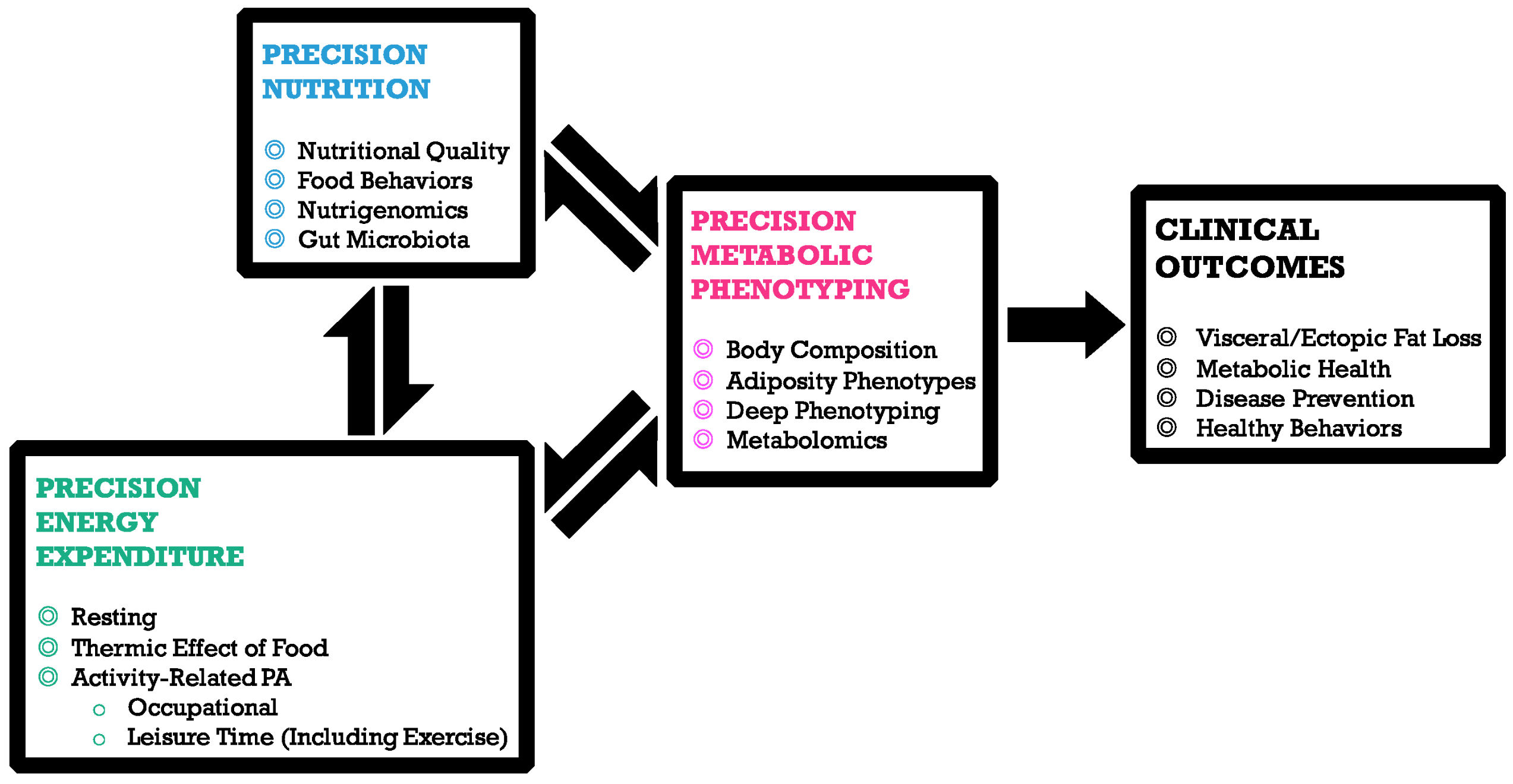
Figure 3: Role of Precision Nutrition
Complex diseases, like diabetes, cancers, cardiovascular and neurodegenerative disorders, are multifactorial - and various genetic, environmental and lifestyle factors needs to be looked at to decrease the risk of these disorders.
Many people today select a diet, such as keto diet, plant based or mediterranean diet in order to lose weight and stay fit. But as mentioned earlier, the long-term practice of a diet that eliminates food groups can lead to malnutrition and deficiency in various essential micronutrients.
The genetic makeup of an individual to metabolize omega-3 is crucial as variants in the FADS1 gene can affect the levels of omega-3 fatty acid in the blood.
Similarly, individuals who follow ketogenic diet are at risk of developing metabolic disorder related to saturated fat if they carry genetic mutation in any of the genes involved in the metabolism of fats and lipids. Some of the genes that are involved in lipid and fat metabolism include PPARG, FTO, APOA2, and ADRB2.
The presence of a mutant variant of these genes in an individual who may follow a keto diet are at risk of obesity as these variants may be unable to metabolize the stored fats in the tissues.
So, the whole point of adapting a tailored or customized diet is to know your metabolism and your genes. Without the precise knowledge of your genome vulnerabilities and your metabolic shortcomings, the food and nutrients we intake can do more damage than good to our body.
Is ideal nutrition the solution to all chronic diseases?
The human body is an amazing machine. It is also very unpredictable at times becuase we have barely begun understanding it. Look at genetics, although we have lots of genome wide association studies, we need more randomized trials involving multiple ethnicities to figure out the underlying causes behind a lot of diseases. Especially if they are polygenic. We've only just begun scratching the surface.
The science of nutrigenomics, nutrigenetics and epigenetics - the interaction effects of genes in relation to food is crucial for the prevention and control of chronic disease. But is this knowledge and understanding enough? We would argue that it is only one side of the equation.
Chronic diseases are far more complex and are influenced by factors more intricate than just food and genes. Various environmental factors such as presence of toxins, and chemicals in the food and in our environment can take a toll on our health without us knowing the exact cause.
Lifestyle is another major health determining factor. Leading a physically active and stress-free life can have positive effects on our mind and body. Exercise changes us at the molecular level and helps us go through healthy aging. Eating patterns, sleeping routine and work habits are other factors that influence our life and wellbeing.
Nutrition is however one big part of the equation, an equation that is complex and evolving.
A deeper understanding of our genetics has given us a powerful tool in our arsenal for war against chronic illnesses plaguing humanity. It gives us an edge by showing us a way to understand our bodies and unique health risks better, so that we can tailor our nutrition better.
We at Gini Health believe that the right food, at the right time, in the right amount can give our cells the right environment to live a long and healthy life - and this in turn can increase human healthspan and lifespan.
That’s the ideal we’re striving towards.



